COVID-19 has wreaked enough havoc, and it still hasn’t subsided. Parallelly, a number of national and international initiatives are going on to develop and mass-produce an effective vaccine to counter the invisible enemy. Some clinical trials are underway, but realistically speaking, it may take a couple of years to build a foolproof COVID-19 vaccine.
What is the current status of COVID-19 vaccine development?
The number of vaccine candidates is quite encouraging, as there are more than a hundred, in pre-clinical stages, with pharmaceutical companies, academic institutions, and government agencies working in tandem. More than seventy of these are being tracked by the World Health Organization (WHO). Several of these candidates are showing a lot of potentials, but experts warn that we shouldn’t get too ahead of ourselves as we are still in quite an early stage.
Here are some of the most promising vaccine candidates
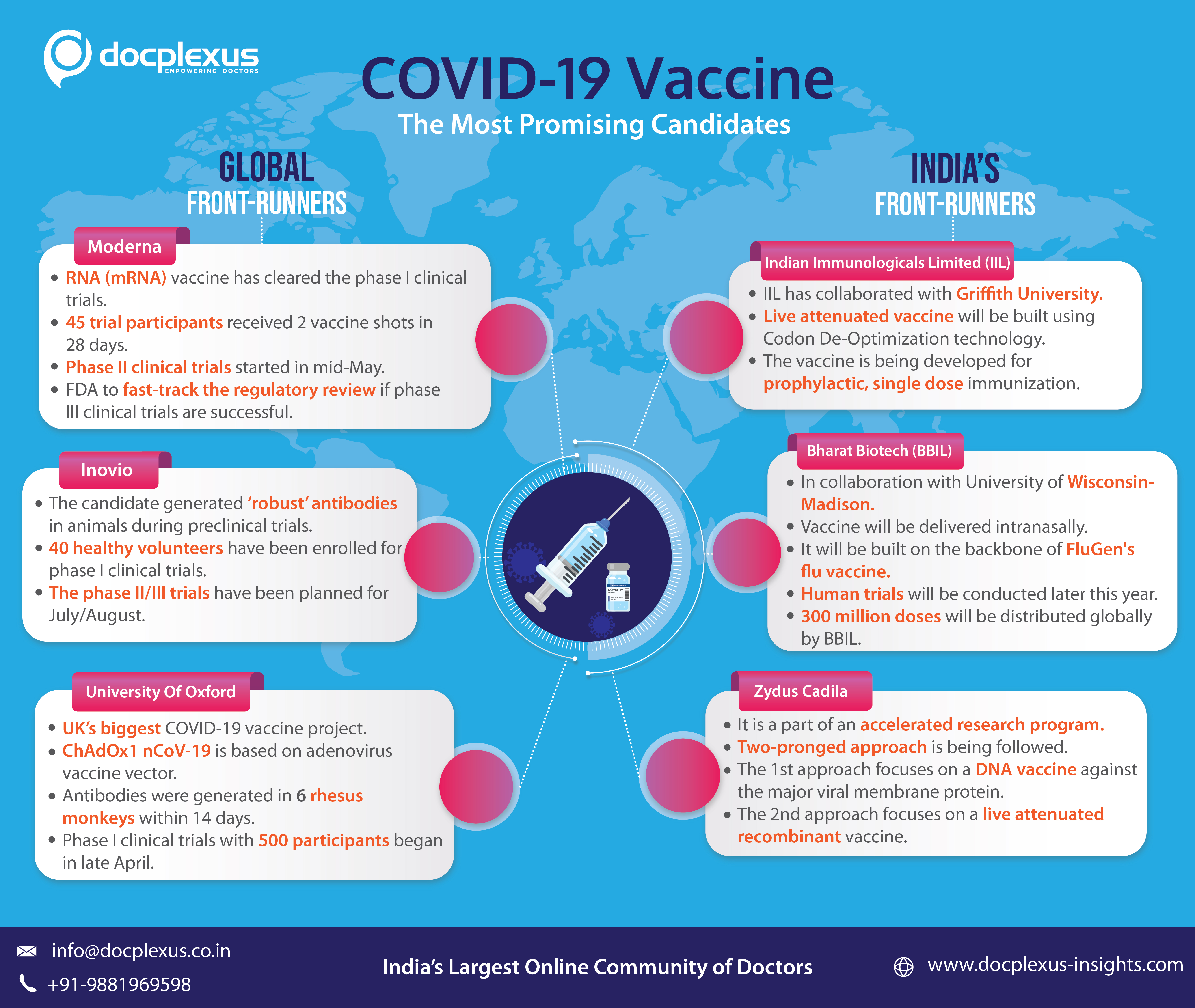
1. Moderna
In phase I clinical trial, the company tested its messenger RNA (mRNA) vaccine, in March. The study included 45 healthy volunteers (ages 18 to 55) who received two shots in 28 days. The results were promising as the vaccine produced antibodies in all 45 participants.
After the nod from FDA, phase II clinical trials began in the mid-may. FDA has also agreed to fast-track regulatory review of the vaccine, if the phase III clinical trials are successful.
2. Inovio
The company was already working on a DNA vaccine for MERS when COVID-19 surfaced in December. This helped them to quickly develop a potential vaccine for SARS-CoV-2, using its suitable DNA medicine platform. The candidate generated robust antibody responses in animals during pre-clinical trials.
At the end of April, 40 healthy volunteers were enrolled to test the vaccine in phase I clinical trial. 94% of them demonstrated overall immune responses at week 6 after two doses. The phase II/III trials are slated for July/August, 2020.
3. The University of Oxford in England.
It is the UK’s biggest COVID-19 vaccine project. The vaccine (ChAdOx1 nCoV-19) is based on the adenovirus vaccine vector and the SARS-CoV-2 spike protein. In the pre-clinical trials, six rhesus monkeys were administered with the vaccine, and antibodies were generated in the animals within 14 days. A clinical trial with more than 500 participants began in late April.
The university has partnered with the Indian Serum Institute, AstraZeneca, and five other global institutions for developing and distributing the vaccine across the world.
How’s India faring in COVID-19 vaccine development?
According to India’s Principal Scientific Advisor, Prof K Vijay Raghwan, the country’s efforts to develop the COVID-19 vaccine will cost USD 2-3 billion.
Currently, India is participating in a variety of COVID-19 vaccine development initiatives. Some initiatives are fully indigenous while some are in collaboration with foreign players where we are playing second fiddle.
Currently, we have 14 vaccine candidates that are working on different levels. Here are the vaccine candidates that may enter the clinical trial stage in the next three to five months.
1. Indian Immunologicals Limited (IIL)
The company has collaborated with Griffith University, Australia, to develop a Live Attenuated Vaccine. The partners intend to use codon de-optimization technology, which would offer sustained protection with a single dose. As per IIL, the technology is highly suitable for developing a vaccine for prophylactic, active, single-dose immunization against COVID-19.
Once Griffith University is done with the research, the vaccine strain will be transferred to IIL, which will then take responsibility for further development and clinical trials. It will be using its Vero cell platform technology to manufacture the vaccine at a large-scale.
2. Bharat Biotech (BBIL)
Bharat Biotech is developing and testing a vaccine called CoroFlu in collaboration with the University of Wisconsin-Madison and FluGen. The vaccine will be delivered intranasally and built on the backbone of M2SR (FluGen’s flu vaccine). The human trials will be conducted later this year. BBIL will manufacture the vaccine, conduct clinical trials, and prepare to produce almost 300 million doses of vaccine for global distribution.
3. Zydus Cadila
The company has initiated an accelerated research program with multiple teams in India and Europe. It is developing a vaccine based on two approaches.
The first approach is focusing on developing a DNA vaccine against the major viral membrane protein, which is responsible for the cell entry of COVID-19.
The second approach is centered around a live attenuated recombinant (measles virus vectored) vaccine against COVID-19 that is expected to induce long-term neutralizing antibodies.
Human Challenge Trials – A way to expedite vaccine development?
Some medical scientists are in favor of conducting Human Challenge Trials, to speed up the vaccine development. In such trials, healthy volunteers are intentionally infected with the virus before getting administered with a potential vaccine candidate. More than 16,000 people across 100 countries have signed up for participation.
However, there are ethical questions surrounding such trials, especially in the context of COVID-19. The medical fraternity still doesn’t know much about the coronavirus, and therefore, no one can pinpoint the risks of participating in a Human Challenge Trial. As a result, participants can’t give informed consent either, which is an essential part of modern clinical trials. But given the nature of the pandemic, some experts feel that such kind of trial is inevitable.
In this regard, the WHO has released ethical guidelines to help the medical fraternity navigate these tricky waters.
Conclusion
In the middle of these extraordinary efforts to secure a vaccine, scientists are still investigating the behavior of the novel coronavirus, and they are trying to understand how effective a vaccine will be against this mutating virus. The mutations may not necessarily make the virus more lethal or infectious, but still, uncovering such details will only help in the development of a successful vaccine.
Our research team leaves no stone unturned in sharing latest insights with our pharmaceutical industry partners. Not just that, we strive towards innovating their communication to HCPs, with our cutting-edge digital solutions. To know about our digital solutions, you can reach us at: info@docplexus.co.in/ +91 9881969598
Sources –
Docplexus – Pharma’s Trusted Marketing Partner
Docplexus is the largest digital network of doctors in India & a trusted marketing partner of pharma & medical device companies. We empower our industry partners to meaningfully engage with the medical community through data-driven, evidence-based marketing & brand management solutions such as Infocenters, Mindset Analysis, KOL Webinars, Sponsored Medical Updates, and Online CMEs.
