
The COVID-19 outbreak has not only affected the global clinical scenario but is also limiting the overseas supply of several drugs manufactured in India. On 3rd March 2020, a committee appointed by the Government of India announced restrictions on the export of key drugs that use active pharmaceutical ingredients (API) imported from China. This move is a bid to secure sufficient supplies for the Indian population.
What is the exact scope of drug export restriction?
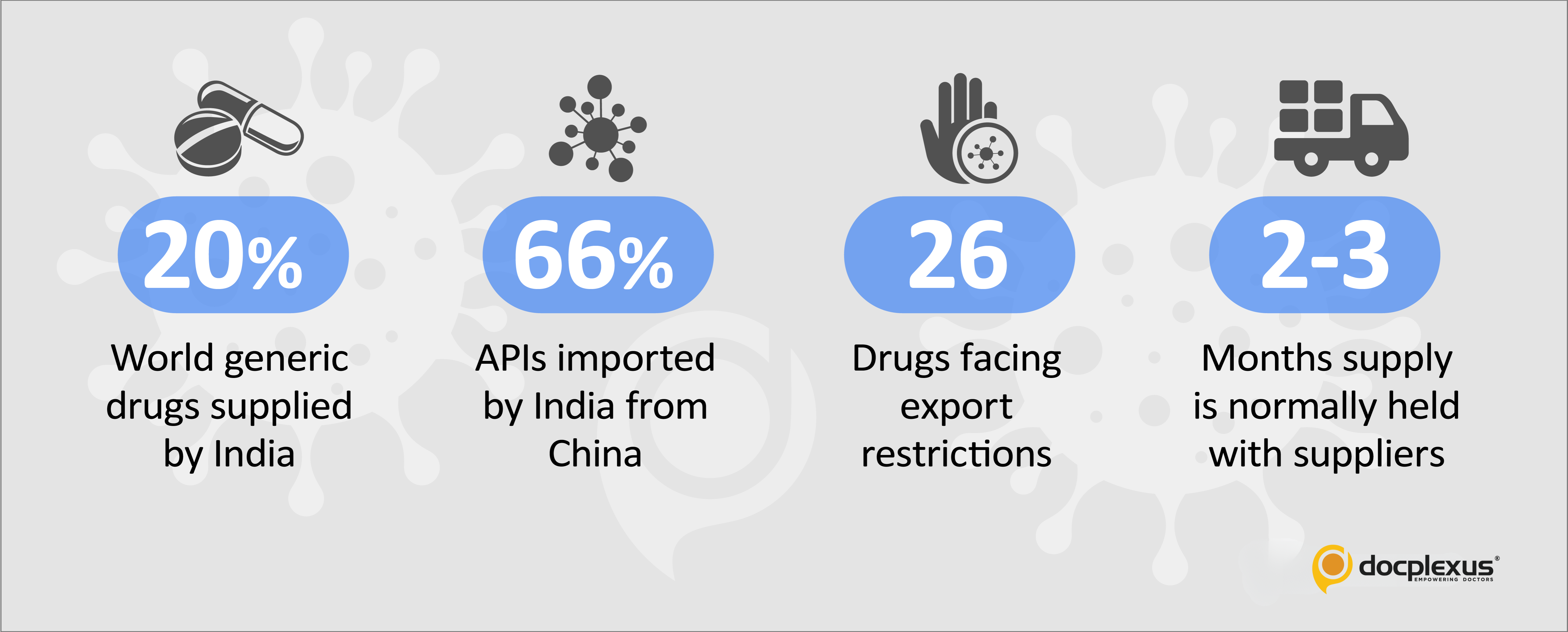
Department of Pharmaceuticals (DoP) has identified 26 drugs exported extensively from India that are particularly at risk of running out of stock since the bulk supplies of their APIs from China have been disrupted.
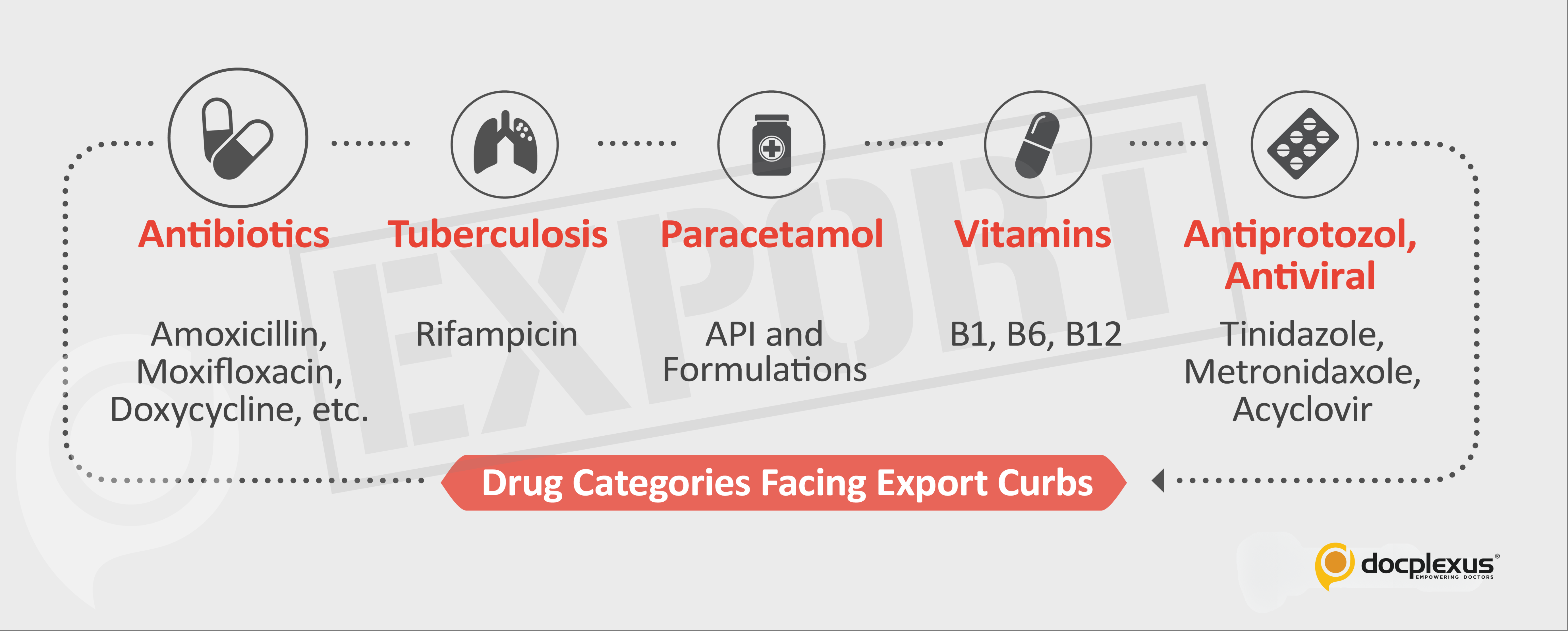
Circular 50, dated 3rd March 2020, “Amendment in Export Policy of APIs and formulation made from APIs” published by DGFT is the primary official document stating the restricted export of drugs from India.
Circular 48, DGFT dated 25th February 2020, prohibited the export of all protection equipment including clothing and masks, goggles, shoe covers, ophthalmic instruments, breathing appliances, tarpaulins, and Biopsy Punch. This hints towards the large scale of precautionary majors being taken by the Government.
What do the experts have to say?
According to an official of the 8-member Government committee, “We have gathered that there is enough inventory to continue for at least the next two months. However, since Hubei and Shandong are the two provinces that supply 20-25% of the raw material of 12 essential drugs, if the lockdown continues for another 15 days, there may be a cascading effect.”
Dinesh Dua, Chairman, of the Pharmaceuticals Export Promotion Council of India (Pharmexcil), told Reuters, “Irrespective of the ban (restrictions), some of these molecules may face shortages for the next couple of months.“
Ranjit Shahani, former head of Novartis India, noted, “A potential shortage of critical medicines is projected, which demands an affirmative action to ensure that supplies are available to Indian citizens”. He further added that a possible escalation of the situation may lead to a total lockdown of the supply system, impacting the whole world.
Also Read: A Guide to Potential Drugs Against COVID-19
The official website of the Commerce Ministry reports USA, the UK, and South Africa as the top Pharma export destinations for drugs made in India with about 12% rise recorded in the past 3 years.
Collateral damage
Exporters and manufacturers are concerned about the threat of procurement agencies blacklisting them due to a lack of supply. To protect their interests, Pharmexcil, which functions under the Commerce Ministry, has requested the DGFT to allow the export of drugs that were already manufactured and made ready for export at ports before the Government imposed the restrictions.
A sharp increase in drug prices by bulk API traders seems to be an inevitable consequence of the viral outbreak. Economic Times has already reported a price hike of about 10-15% and, in some cases, even up to 50% for different drugs.
There is rising concern over the dependence of world economies on China, with experts noting that the Chinese APIs shortage is only the latest example after iPhones and auto parts.
Mansukh Mandaviya, Minister of State for Chemicals and Fertilizers however assured that the export curbs would be temporary – “It is not a permanent measure and is being reviewed on a daily basis.”
COVID-19 – India Count
50+ people have been afflicted with coronavirus in India so far, The DoP committee chairman Dr. Eshwara Reddy, Joint Drugs Controller, Central Drugs Standard Control Organization (CDSCO) has initiated assessment and action against the deadly virus.
Ms. Preeti Sudan, Secretary, Health & Family Welfare, has also confirmed that the Ministry of Health and Family Welfare is striving to maintain a high level of vigil and alertness against COVID-19.
Source: New York, Economic Times, Times of India, Directorate General Of Foreign Trade website, Press Information Bureau website, India Today, Ministry of Commerce and Industry- Department of Commerce website, Department of Pharmaceuticals website, Directorate General of Foreign Trade website, Fortune Media IP Limited.
Also Read: A Guide to Potential Drugs Against COVID-19
Docplexus is one of the world’s largest & fastest-growing networks of verified doctors and a trusted marketing partner of pharma, medical devices, diagnostics & nutraceutical companies. We empower our industry partners to meaningfully engage with the medical community through data-driven, evidence-based marketing & brand management solutions such as infocenter (branded microsite), mindset analysis, KOL webinars, sponsored medical updates, online CMEs & more.
