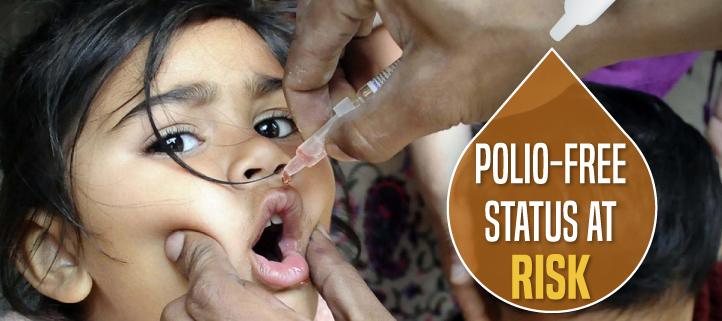
The contamination of nearly 1.5lakh vials of polio vaccine has raised doubts over the quality of pharma products in India. The Drug Controller Authority of India (DCGI) on Wednesday has ordered the Central Drug Laboratory in Kasauli to test every batch of polio vaccine for contamination.
The contaminated vials were manufactured by a Ghaziabad-based company and its managing director was arrested last week. Authorities initiated a probe at the premises of the company on Thursday to identify the source of contamination. A four-member team inspected the packaging and manufacturing process at the company.
While officials are trying to establish the source of contamination, it is important to note that the National Institute of Virology in Pune is the only laboratory authorised to keep stock of wild and vaccine strains of polio type 2 virus.
India was declared a polio-free nation in 2014. The last case was reported in 2011 in Howrah, West Bengal. India had nearly eliminated type 2 polio virus by 1999, but its presence continued in a few samples of the trivalent vaccine. Later, India, along with the entire world, switched to bivalent vaccines in 2016.
Around 15 days ago, World Health Organization (WHO) tested stool samples of patients from 45 sites across 8 states. A few people were reported to have showed paralysis and polio-like symptoms. And, the results confirmed contamination with type 2 virus. Every year, WHO collects nearly 75,000 samples and tests in laboratories across India as a part of its routine surveillance.
WHO has stepped up its surveillance across the country. The Union health ministry has also chalked out an intense action plan to ensure no children are injected with the infected polio vaccines. All states have been put on high alert. The contaminated vials were sent to Uttar Pradesh, Maharashtra and Telengana. The health ministry on Wednesday issued a statement clarifying that there is ‘practically nil’ chances of any child getting infected by polio.
The incident has instilled fear among people, and they are now skeptical about vaccinating their children. It has also increased the burden on doctors, as they will now struggle to ensure no contaminated vaccines are administered to children. A news report stated that children born after 2016 are not immune to type 2 polio virus.
Recently, another company was charged with manufacturing faulty hip replacement systems. The company had to recall the product across the world.
Regulatory authorities like the U S Food and Drug Administration (USFDA) have efficient monitoring mechanisms to ensure pharma maintains quality of its products. In the event of lags, USFDA issues strict warnings and the actions include recalling the batch of products, ban on the product, heavy penalty, etc.
Share your thoughts on how Pharma can prevent contamination while manufacturing drugs and vaccines
Source: The Times of India, The Indian Express, livemint.com, The New Indian Express & The Financial Express
The recent events that raised doubts over pharma’s quality standards have led to the erosion of doctors’ trust for pharma. To secure physician’s trust read this article.
