
Having gained a strong foothold in the generics market, Indian pharma industry is now looking to establish itself as an innovation hub by pioneering research into path-breaking therapies. This, coupled with the profound paradigm shift from experience-based to evidence-based medical practice, establishes Clinical Trials as a critical component of healthcare in coming years.
India is globally recognized as an attractive destination for conducting clinical trials owing to its huge patient pool, a technically competent workforce and significant cost savings. However, it accounts for less than 1.4% of global clinical trials at present. While the government is already working on the regulatory environment to increase these numbers, it will be equally important to encourage higher participation from the medical community.
Physician-researchers play a huge role in improving the quality of both services and research studies. In India, the linguistic barrier is a major problem for recruiting patients and giving them proper instructions and knowledge about trials. Hence, it is of utmost importance for physicians from all across the country to take part in clinical trials. Since a majority of trials are industry-sponsored, it is crucial for pharma to examine and eliminate factors that hold physicians back from being actively involved in regulatory studies.
Docplexus conducted a survey on 1105 doctors specializing in Pharmacology/Clinical Pharmacology, General Medicine, Cardiology, Orthopedics, Neurology and Urology to understand their inclinations and concerns regarding clinical trials in India.
It revealed following key insights:
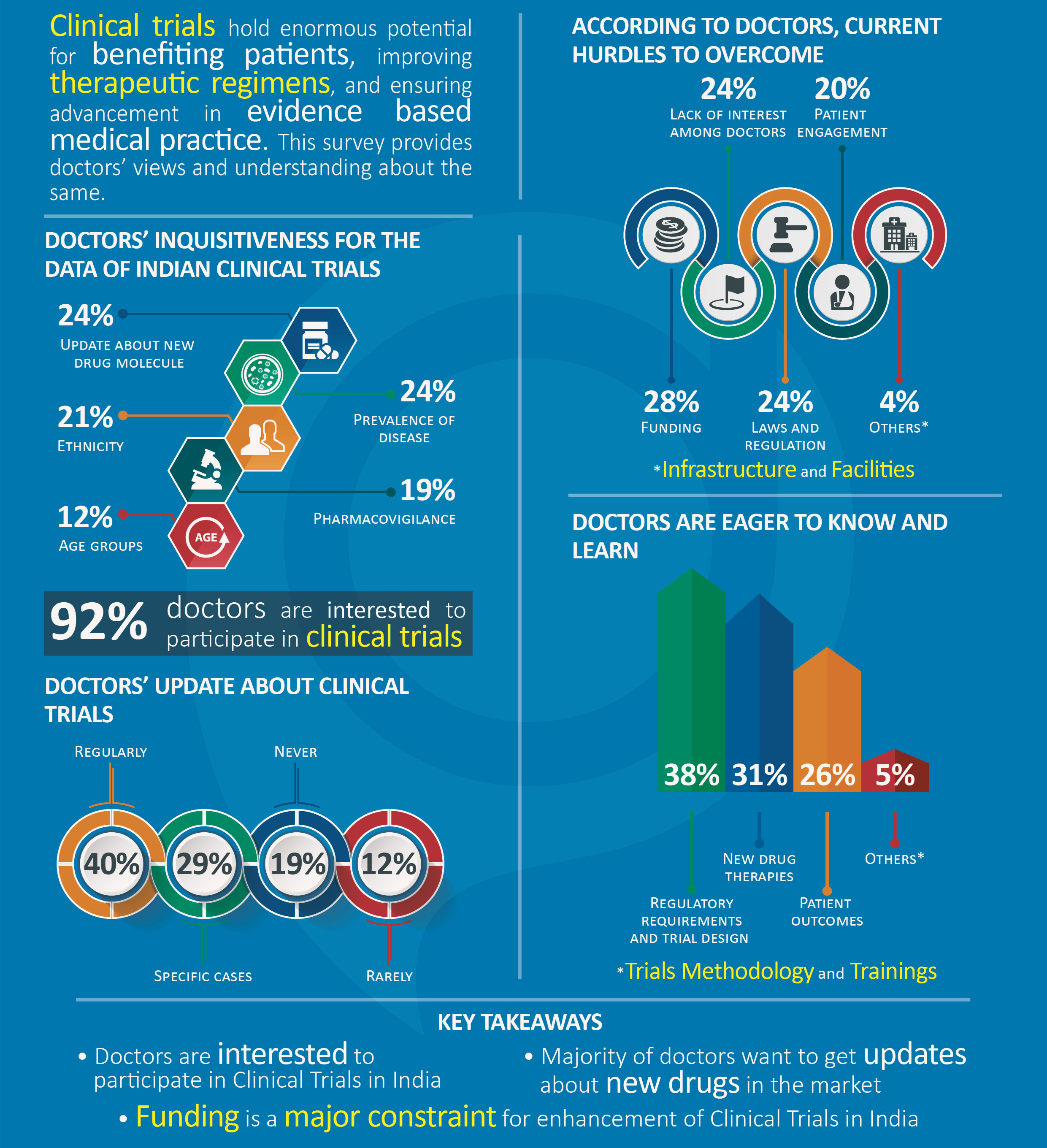
- Doctors Are Interested in Clinical Trials Data: Clinical trials are the cornerstone of progress in disease treatment. Upgrading the clinical practice with latest evidence-based medicines could improve patient outcomes. Doctors are eager to know about latest drugs in the market or their current efficacy to treat a particular disease. Ethnicity does matter because a different individual may respond differently to a particular treatment. These are the main reasons behind doctors’ overall inquisitiveness in clinical studies.
- Doctors Want to be Updated on Clinical Practices: As stated earlier, physicians’ participation would enhance and improve the clinical research. The survey reveals high inclination of doctors towards clinical trials. About 92% of Indian physicians are willing to participate in clinical trials and work for the advancement of medical knowledge. Besides this, most doctors (40%) regularly update themselves with latest clinical practices. Although 12% stated that they rarely accessed clinical trial information, this number should decline when such information becomes easily available on digital media. This data has positive implications for the pharma industry and subsequently, Indian healthcare.
- Several Barriers Restrict Physicians’ Participation in Clinical Trials: Physicians’ reluctance towards participation in clinical trials may due to a variety of factors. For instance, a review suggests that factors such as lack of time and resources, communication difficulties, conflicts between the role of clinician and scientist, inadequate experience and training, lack of rewards and recognition for physicians, etc. can cause a physician to step back. 28% of doctors feel that funding or financial assistance is a major obstacle to physicians’ involvement in the clinical trials. Patient engagement for a clinical trial is another major concern for both physicians as well as pharma. This is evident from a study which demonstrates that 50%–80% of eligible patients are not recruited in clinical trials because of their doctors’ decision to not offer the trial to that patient.
- Poor Access to Latest Practices is Creating Misconceptions: The survey analysis specifies that 38% of doctors want to acquaint with current laws and regulations followed by knowledge about trial design. In reality, the medical education system hardly puts an emphasis on regulatory studies in their academic syllabi and medical students don’t get enough knowledge about clinical trial methodology. This may have relevance to a common misbelief in India that the human participants are treated as laboratory animals in clinical trials. In fact, there are strict guidelines in place to ensure that patients and all other clinical trial volunteers are treated fairly and ethically. As per the new regulations, before an investigational drug can be given to participants in clinical trials, scientists must complete a rigorous screening and preclinical testing process, which can take up to six years to complete.
It is evident that physicians acknowledge the huge role played by clinical trials in improving healthcare outcomes. Their participation will only increase as they get adequate financial assistance, protected time for research, help with data management and regular updates on regulations and practices.
Key Takeaways for Pharma
- Doctors track clinical trials data to gauge the prevalence of disease, stay updated on new molecules, and understand the role of ethnicity in patient’s responsiveness to treatment.
- Pharma should encourage the interest of physicians to participate in clinical trials by giving them adequate financial assistance.
- There should be a system or information portal in place to educate and train the doctors about regulations and trials methodology in clinical trials.
Docplexus hosts a dedicated Infocenter named ‘World of Clinical Research’ through which it updates doctors on latest clinical trial practices.
